Table of content
Press Release: 25th April 2023.
Omnipod GO is a self-contained insulin delivery system that can be worn and provides continuous rapid-acting insulin at a fixed rate for up to 72 hours.
This latest addition to the Omnipod product line features a tubeless and waterproof* Pod and is available at seven different pre-programmed daily rates, ranging from 10 to 40 units per day. The device does not require a handheld device to control the Pod.
It is cleared for use with U-100 insulins, including NovoLog®, Fiasp®, Humalog®, Admelog®, and Lyumjev®.
Insulet Corporation announced the FDA clearance of its latest innovation, Omnipod GO™, an insulin delivery device cleared for use for people with type 2 diabetes age 18 or older who would typically take daily injections of long-acting insulin.
Insulet plans to commercialize Omnipod GO in the United States in 2024.
About Insulet Corporation:
Based in Massachusetts, Insulet Corporation (NASDAQ: PODD) is a medical device company that strives to simplify the lives of people with diabetes and other conditions through its innovative Omnipod product platform.
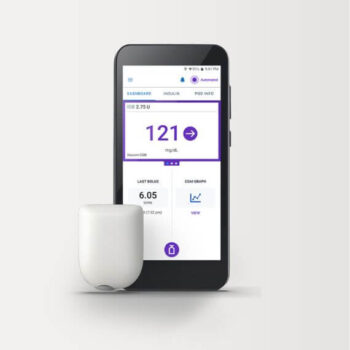
The Omnipod Insulin Management System offers a tubeless disposable Pod that can be worn and delivers non-stop insulin for up to three days without the need for needles.
Insulet flagship innovation, the Omnipod® 5 Automated Insulin Delivery System, is a continuous glucose monitor that manages blood sugar without the need for multiple daily injections or finger sticks.
It can be controlled by a compatible smartphone or Omnipod 5 Controller. Insulet also uses its Pod design to tailor its Omnipod technology platform to deliver non-insulin subcutaneous drugs for other therapeutic areas.
Reference: INSULET PRESS ROOM














Write a comment
Your email address will not be published. All fields are required