Medicare coverage is now offered!
Press release: July 10, 2023
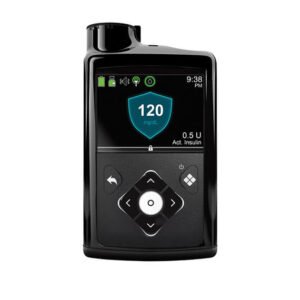
Medtronic’s MiniMed 780G system, with the Guardian 4 sensor, is now covered by Medicare in the US. This advanced system adjusts insulin based on CGM readings, helping manage diabetes better.
In April 2023, the FDA approved the Medtronic MiniMed 780G insulin pump for people with type 1 diabetes aged 7 and above. This allowed Medtronic to start sending out the new pump across the U.S. The pump had faced delays in getting FDA approval due to quality control problems at Medtronic’s manufacturing site, after being submitted for approval in 2021. It had already been approved in Europe in 2020 and in Canada in November 2022. Now, shipments of the MiniMed 780G are finally on the way to help manage diabetes for those who need it.
What is MiniMed 780G automated insulin pump?
The MiniMed 780G is an innovative medical device equipped with SmartGuard technology, removing the requirement for fingerstick tests. It boasts Meal Detection Technology that makes continuous adjustments to sugar levels every five minutes. This feature applies to both the background insulin (basal) and the mealtime insulin (bolus) needs, ensuring more precise diabetes management. This advanced system enhances convenience and accuracy by automatically responding to glucose fluctuations, streamlining insulin delivery for individuals using the MiniMed 780G.
Reference: Medtronic’s MiniMed 780G Automated Insulin Pump Now Covered by Medicare














This page is extraordinary. The magnificent data uncovers the proprietor’s responsibility. I’m stunned and sit tight for additional such marvelous posts.
Hello my loved one I want to say that this post is amazing great written and include almost all significant infos I would like to look extra posts like this