Table of content
Since years, continuous glucose monitors (CGMs) could only be obtained by prescription and were mainly used by individuals with Type 1 diabetes or by individuals who require insulin. However, today, there is an innovative new product that is changing that image by Abbott. The proposal is to introduce the FDA-approved over-the-counter Libre Rio, which is a device aimed at adult patients with Type 2 diabetes who do not require insulin to control their condition.
This move is not only another technological update but a massive change in the direction of accessible and real-time glucose monitoring by millions of individuals. Now we will look at how the Libre Rio CGM functions, why it is so unique and how it can revolutionize your everyday routine in managing diabetes.
About Libre Rio:
Ages 18 and over who do not use insulin are the target market for the over-the-counter integrated continuous glucose monitoring (iCGM) gadget called Libre Rio. You run the risk of missing a severe low or high glucose incident if you don’t use Libre Rio according to the label instructions. When making treatment decisions, consider the fingerstick value obtained from a blood glucose meter if readings do not correspond with symptoms or expectations.
FDA Approved OTC Libre Rio:
Abbott launched an innovative device called Libre Rio, a CGM for persons with Type 2 diabetes who do not apply insulin. The Abbott Libre Rio is FDA-approved to be used in persons with type 2 diabetes who are at a minimum of 18 years old. Adults who control their diabetes by lifestyle changes and do not take insulin are the target market for the over-the-counter CGM. Although Abbott kept the research under wraps, it is now prepared to take on Dexcom, which was approved in March as the first over-the-counter continuous glucose monitor for diabetics who do not use insulin.
Note: The FDA clearance (510(k) K233861) confirms that Libre Rio meets strict accuracy and safety standards under FDA regulation 21 CFR 862.1355. It’s classified as a Class II medical device, cleared for use in non-insulin adults aged 18 and older.
First over-the-counter CGM:
According to Abbott, Rio is the first over-the-counter CGM with a measuring range of 40 to 400 mg/dL that enables the monitoring of exceptionally high or low glucose episodes. Libre Rio will become a part of Abbott’s larger Libre line of CGM devices, which are now utilized by over 60 million individuals worldwide2. The Freestyle Libre 2 and Freestyle Libre 3 systems are part of the Libre group in the United States and are proposed for individuals with Type 1, Type 2, and gestational diabetes.
Lisa Earnhardt, group president of Abbott’s medical devices division, said in a report that “there is no one-size-fits-all method for glucose monitoring, which is why we’ve intended distinctive products for diverse people — all built on the same world-leading bio wearable technology.” “Some elements are essential for people with diabetes, such as data exchange with healthcare providers or prescription tracking. To maintain their metabolic health, those without diabetes require various elements, such as individualized coaching to encourage practical lifestyle modifications.
Prescriptions are needed for Freestyle Libre systems, and the majority of major U.S. insurance covers them, too. Libre Rio may facilitate the use of a CGM by Americans with diabetes and help them track their progress toward health objectives. It also provides them with info to share with their healthcare doctor when they next officially visit them.
Components of Libre Rio Sensor
Based on the Abbott Freestyle Libre CGM, the system contains a biosensor that is worn on the back of the arm for a maximum of 15 days, and a reader or well-matched smartphone app that shows the user’s blood glucose levels. The Libre Rio has an amount range of 40 mg/dL to 400 mg/dL, allowing for the measurement of very high or low glucose levels. There are two main categories based upon the division of the components of Libre Rio, including:
Built-in Sensor Components
- Filament: It is a tiny, flexible sensor filament that is inserted just under the skin to measure glucose in the interstitial fluid and measure the glucose level accordingly.
- Battery: A built-in battery that charges the sensor for its 15-day life.
- Transmitter: The sensor transmits data via Bluetooth Low Energy (BLE).
External Components
- Sensor Applicator: A single-use device used to apply the sensor to the skin, which includes an electron beam-sterilized sub-component. You sound like a “Click” for proper sensor application.
- Libre Rio Mobile Application: A smartphone app (download on the Play Store and Apple Store) that acts as the receiver, displaying real-time glucose readings, trend arrows, and historical data.
Expert Tips: Always download the latest version of the app on the dedicated official platform. No need to download a third-party app or a cracked version, as it will mislead your glucose readings.
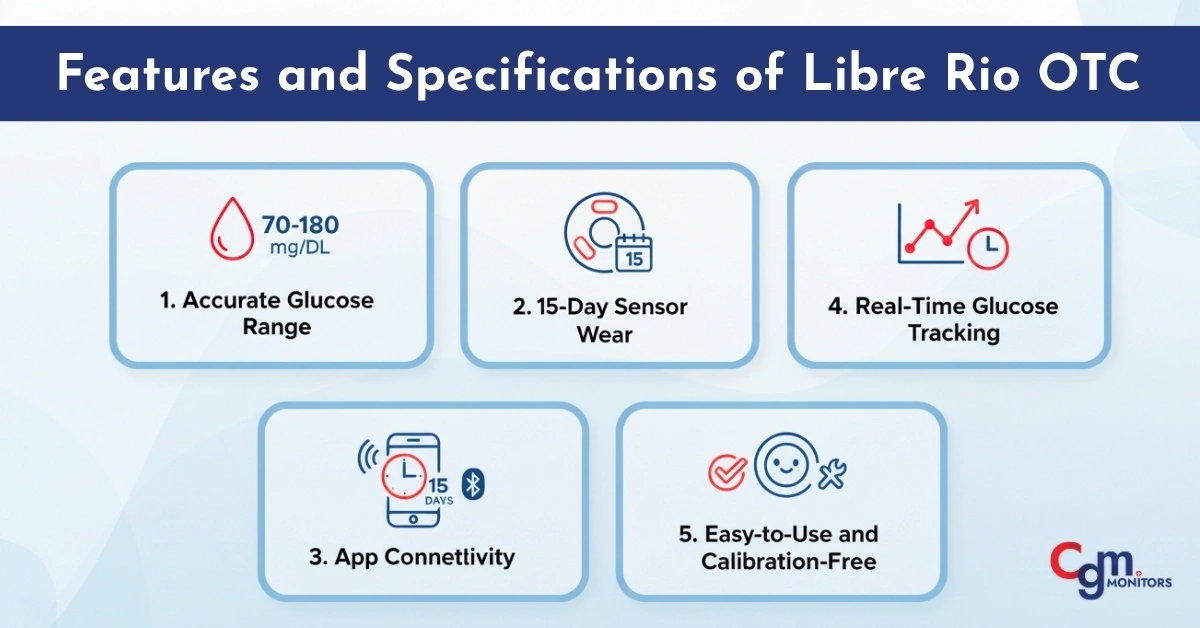
Features and Specifications of Libre Rio OTC
Abbott’s Libre Rio Continuous Glucose Monitor integrates innovation and simplicity in one sleek system. Some of the standout features include:
1. Accurate Glucose Range
The Libre Rio sensor measures glucose within a wide 40–400 mg/dL range, helping users monitor both low and high extremes accurately.
2. 15-Day Sensor Wear
Each Libre Rio 15-day sensor provides continuous readings for up to two weeks, minimizing the need for frequent sensor replacements.
3. App Connectivity
Data from the Libre Rio sensor syncs seamlessly with the Libre Rio app, allowing users to check readings anytime. You can view trends, receive alerts, and share reports directly with your healthcare provider.
4. Real-Time Glucose Tracking
Unlike traditional glucose meters, the Libre Rio CGM gives instant insights into your body’s glucose response, providing trend arrows that indicate whether your glucose is rising or falling.
5. Easy-to-Use and Calibration-Free
The system is completely calibration-free—meaning no fingersticks are required for setup or daily use. This feature ensures a hassle-free, user-friendly experience.
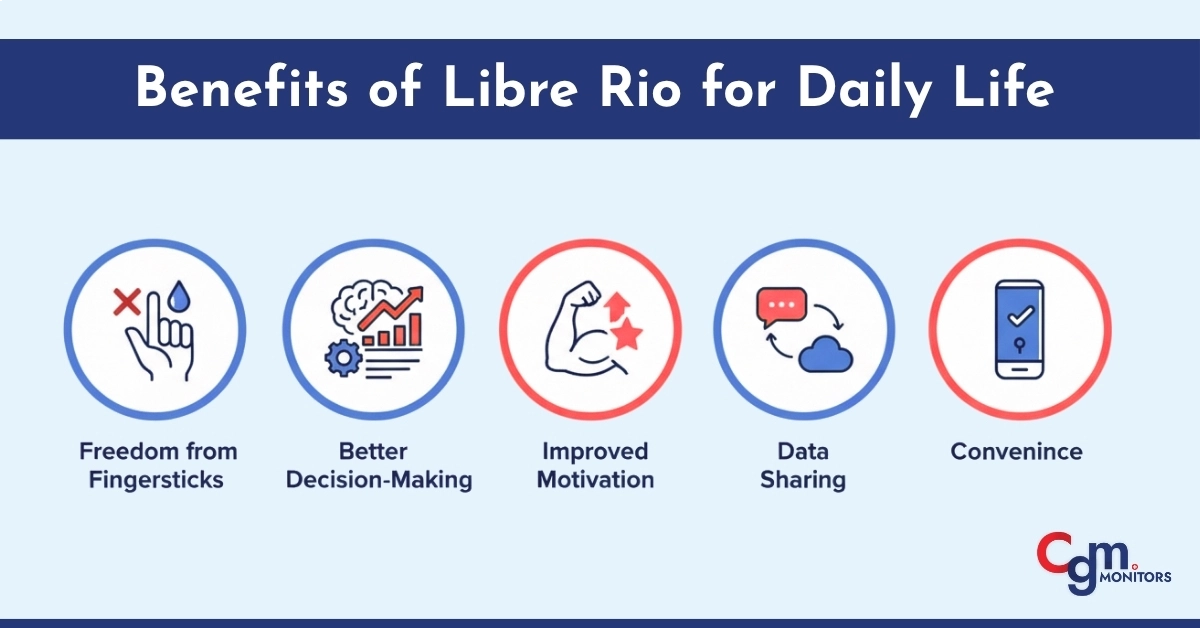
Benefits of Libre Rio for Daily Life
For users managing Type 2 diabetes, integrating Libre Rio into daily life can be truly transformative.
Here’s how it enhances everyday diabetes care:
- Freedom from Fingersticks: No more painful or frequent blood tests.
- Better Decision-Making: Real-time glucose data helps you understand the impact of food, exercise, and sleep.
- Improved Motivation: Continuous feedback encourages healthier lifestyle habits.
- Data Sharing: The ability to share readings with healthcare providers makes checkups more productive.
- Convenience: With over-the-counter access, it’s easy to buy and start using immediately.
Users report that the OTC Libre Rio sensor or prescribed Freestyle Libre 3 Plus not only simplifies glucose monitoring but also reduces anxiety around blood sugar fluctuations. Having constant visibility into glucose trends brings confidence, peace of mind, and control.
Comparing Libre Rio to Other CGMs
The Libre Rio OTC CGM enters the market alongside competitors like Dexcom Stelo and Abbott Lingo—other consumer-focused devices that also received FDA clearance.
While the Dexcom Stelo shares a similar over-the-counter classification, the Libre Rio focuses specifically on Type 2 diabetes management. Meanwhile, Lingo is targeted toward wellness tracking rather than clinical diabetes management.
Here’s how Libre Rio differentiates itself:
- Accuracy: Built on the trusted FreeStyle Libre technology used by millions worldwide.
- Ease of Access: Available without a prescription, making it more accessible than other iCGMs.
- Wear Duration: Offers up to 15 days of continuous glucose tracking.
- Affordability: Expected to be one of the most cost-effective options in the OTC CGM market.
These advantages position Libre Rio as a leader in the next generation of consumer CGM devices.
Conclusion:
Diabetes is among the highest public health challenges in the U.S., with almost 38.4 million people living with the disorder. Freestyle Libre systems have been accessible over the counter in more than 50 countries over the previous decade; but, in the U.S., they have only been accessible through prescription.
Libre Rio may make it easier for Americans with diabetes to try a CGM and start to see advancement in their health goals, allowing them with info to confer with their healthcare provider at their next visit.
Frequently Asked Questions
What is the conclusion regarding Freestyle Libre accuracy?
Understanding the causes of inaccurate readings and implementing possible solutions like regular calibration, proper use, and lifestyle changes can ensure accurate readings and improve the quality of life for diabetic patients.
What is the Over-the-Counter Libre Rio and how does it differ from prescription versions?
The Over-the-Counter Libre Rio is Abbott’s CGM sensor designed for adults with type 2 diabetes who manage their condition without insulin through diet, exercise, or oral medications. Unlike prescription CGMs such as FreeStyle Libre 2, Libre Rio is available without a prescription, offers the same discreet sensor and 14-day wear, and provides a full glucose range of 40–400 mg/dL focused on lifestyle-based glucose management.
How do I purchase the Over-the-Counter Libre Rio without a prescription?
Adults with type 2 diabetes who are not using insulin can purchase Libre Rio over the counter without a doctor’s prescription. It is available through pharmacies, online retailers, and Abbott’s official channels, making access simple for everyday glucose monitoring.
How accurate is the Over-the-Counter Libre Rio for glucose monitoring?
The Over-the-Counter Libre Rio delivers highly accurate continuous glucose readings for daily tracking. It provides minute-by-minute glucose data without requiring fingerstick calibration, helping users identify patterns and make informed diet and exercise decisions.
How does the accuracy of the Over-the-Counter Libre Rio compare to traditional finger-prick tests?
Libre Rio offers a more complete picture of glucose trends compared to traditional finger-prick tests, which only capture a single moment in time. Continuous monitoring helps reduce unexpected highs and lows while eliminating frequent fingersticks.
What are the specific features of the Over-the-Counter Libre Rio compared to the FreeStyle Libre 2?
Libre Rio features the same small sensor size, 14-day wear period, and app connectivity as FreeStyle Libre 2. However, it is available without a prescription, designed specifically for non-insulin type 2 users, offers a broader 40–400 mg/dL range, and focuses on lifestyle insights without real-time alarms.
What age restrictions apply to using the Over-the-Counter Libre Rio?
The Over-the-Counter Libre Rio is approved for adults aged 18 years and older with type 2 diabetes who manage their condition without insulin.
Why is the Over-the-Counter Libre Rio now available without a prescription?
Abbott introduced Libre Rio as an over-the-counter CGM to remove access barriers for adults with type 2 diabetes not using insulin, allowing easier access to continuous glucose insights that support lifestyle improvements and informed discussions with healthcare providers.
When is the best time of day to apply a new Over-the-Counter Libre Rio sensor?
A new Libre Rio sensor can be applied at any time of day that fits your routine. Many users prefer applying it when glucose levels are stable, such as in the morning or evening, to ensure a smooth one-hour warm-up period.
Who can use the Over-the-Counter Libre Rio?
Libre Rio is intended for adults 18 years and older with type 2 diabetes who manage their condition without insulin using diet, exercise, or non-insulin medications
Who should not use the Over-the-Counter Libre Rio?
Libre Rio should not be used by individuals under 18, those using insulin, people on dialysis, critically ill patients, pregnant individuals, or anyone requiring diathermy. Always consult a healthcare provider if unsure.













Write a comment
Your email address will not be published. All fields are required