Table of content
- Why Are These Sensors Failing: The Core Issue
- Why CGMs Matter: The Promise and the Reality
- What Happened: Which FreeStyle Libre Devices Are Affected?
- The Dangers of False Low Readings
- How to Check if Your Sensor Is Affected
- Diabetic Patient Sharing Stories on Forum Platform
- Lessons Learned: The Importance of Glucose Monitor Safety
- Conclusion
- Frequently Asked Questions
In the world of diabetes management, continuous glucose monitors (CGMs) like the Abbott FreeStyle Libre 3 have been a game-changer, offering real-time insights without the constant prick of fingersticks. But on December 2, 2025, a sobering alert from the U.S. Food and Drug Administration (FDA) and Abbott Diabetes Care shattered that trust for millions. Certain FreeStyle Libre 3 and Libre 3 Plus sensors may deliver falsely low glucose readings, potentially leading to dangerous treatment errors. This issue has been tied to over 700 severe adverse events worldwide—and, tragically, seven possible deaths.
The manufacturer Abbott Diabetes Care issued a medical device correction and urged all users to verify their sensor serial numbers for potential replacement.
In this post, we’ll break down the facts, explore user experiences, and provide clear steps to protect yourself. If you’re searching for “FreeStyle Libre 3 false low readings” or “Libre 3 Plus sensor safety alert,” you’ve come to the right place. Let’s navigate this together.
Why Are These Sensors Failing: The Core Issue
Abbott’s investigation pinpointed the problem to a single production line among several used to manufacture Libre 3 sensors. These affected units can report glucose levels that are inaccurately low, misleading users into over-correcting with carbohydrates or skipping insulin doses. The result? Blood sugar mismanagement that escalates to severe complications like diabetic ketoacidosis (DKA), heart attacks, strokes, kidney damage, nerve issues, or heightened infection risks.
The scale is staggering: Approximately 3 million sensors in the U.S. alone are impacted, though about half are already expired or used. Globally, Abbott has logged 736 severe adverse events—57 in the U.S.—and seven deaths, all outside the country. The FDA classified this as a high-risk issue, prompting an “early alert” and urging immediate action. (Source: CBS News)
This is not the first issue reported with the Libre 3. A 2024 recall addressed falsely high readings, and users have long complained about inconsistencies. But the potential for harm here is acute: False lows don’t just annoy—they can cascade into life-threatening events if untreated.
Why CGMs Matter: The Promise and the Reality
Continuous glucose monitors are designed to simplify diabetes management. Unlike traditional finger-prick tests, CGMs use a small sensor under the skin to monitor glucose levels in interstitial fluid — providing frequent, real-time readings, trends, and alerts.
The advantages of CGMs include:
- Continuous tracking of glucose patterns throughout the day and night.
- Insight into current glucose levels and trends (rising or falling).
- Alerts for low or high glucose episodes, potentially preventing severe hypoglycemia or hyperglycemia.
- Integration with insulin pumps to adjust therapy or alert caregivers automatically.
However, CGMs have inherent limitations. Interstitial fluid glucose does not always perfectly match blood glucose levels; sudden changes or tissue conditions can distort readings. Medical guidelines advise that CGM values may need confirmation with finger-prick tests, especially when symptoms do not align with sensor readings.
What Happened: Which FreeStyle Libre Devices Are Affected?
On November 24, 2025, Abbott Diabetes Care issued a “medical device correction” after internal testing showed that some FreeStyle Libre 3 and Libre 3 Plus sensors could give incorrectly low glucose readings.
Details of the Issue
- Affected Models: FreeStyle Libre 3 (Model Nos. 72081-01, 72080-01) and FreeStyle Libre 3 Plus (Model Nos. 78768-01, 78769-01).
- Impact: Approximately 3 million sensors distributed in the U.S., with about half already expired or used.
- Reported Consequences: 736 severe adverse events globally, including seven deaths potentially linked to sensor malfunctions.
- Not Impacted: Libre 3 readers, mobile apps, FreeStyle Libre 14-day, Libre 2, Libre 2 Plus, Libre Pro, and other Abbott biowearables.
| Device | Model Numbers | UDI-DI |
|---|---|---|
| FreeStyle Libre 3 Sensor | 72081-01, 72080-01 | 00357599818005, 00357599819002 |
| FreeStyle Libre 3 Plus Sensor | 78768-01, 78769-01 | 00357599844011, 00357599843014 |
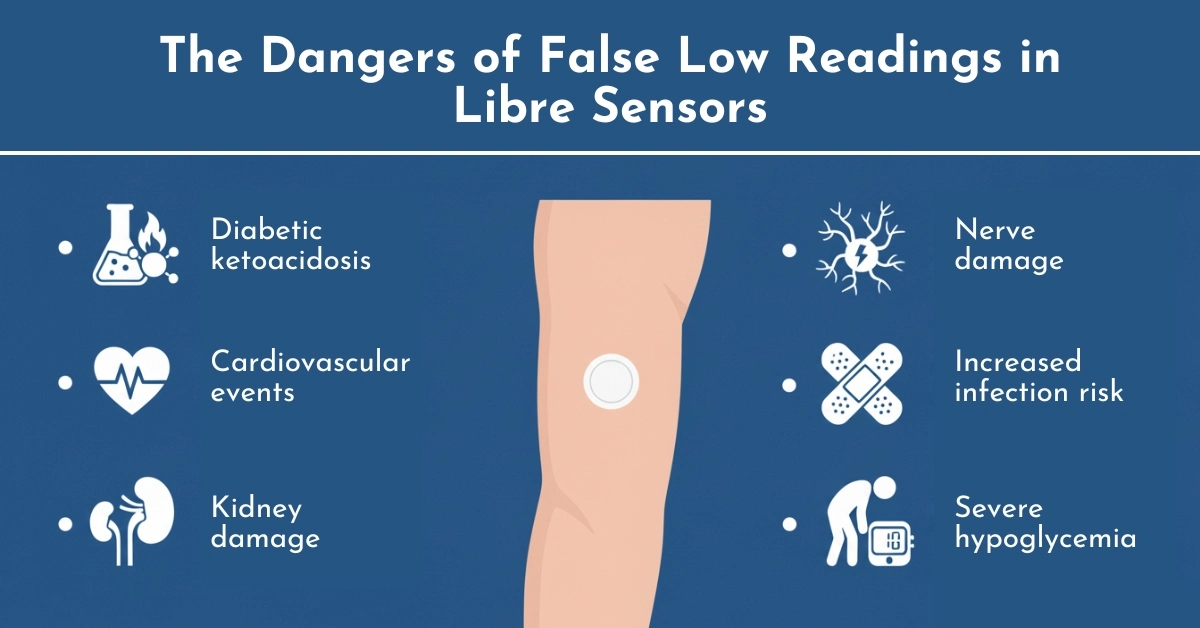
The Dangers of False Low Readings
When a glucose monitor reports falsely low blood sugar levels, users may take actions that actually lower their glucose further, creating a dangerous situation called hypoglycemia. Conversely, if users ignore genuinely low readings thinking their device is malfunctioning, they risk severe hypoglycemic episodes.
Complications from improper diabetes management due to inaccurate readings include:
- Diabetic ketoacidosis (DKA): A serious condition where the body begins breaking down fat too quickly, producing toxic acids
- Cardiovascular events: Including heart attack and stroke
- Kidney damage: Chronic high blood sugar can permanently damage kidney function
- Nerve damage (neuropathy): Leading to pain, tingling, or loss of sensation
- Increased infection risk: Poor glucose control weakens the immune system
- Severe hypoglycemia: Which can cause seizures, loss of consciousness, or death.
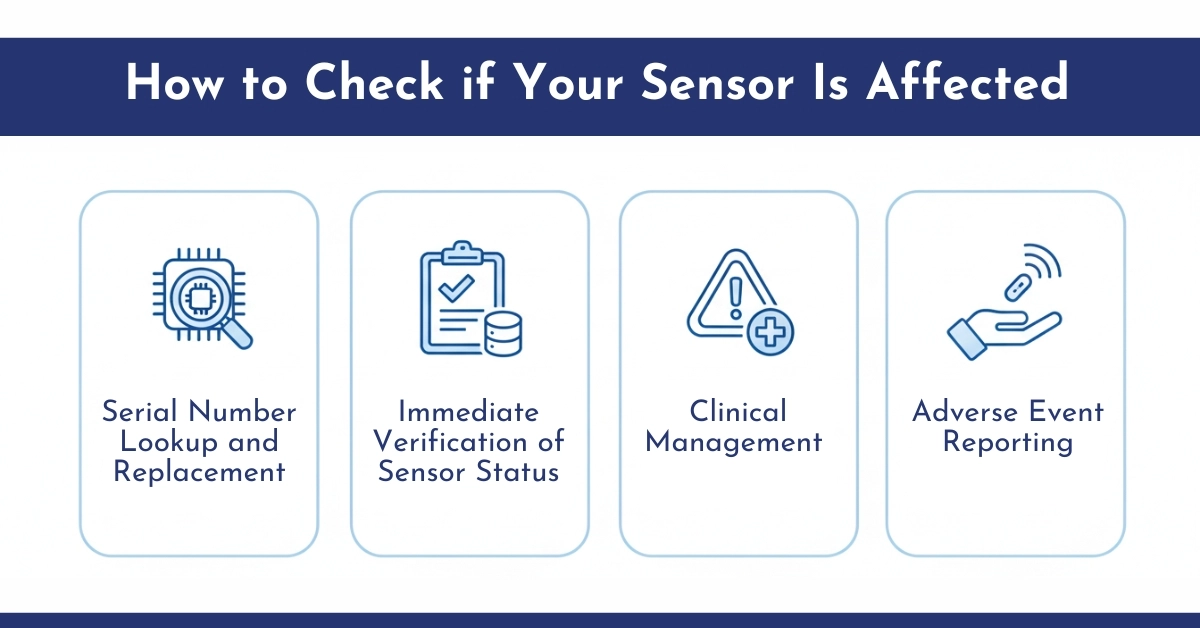
How to Check if Your Sensor Is Affected
Abbott has made it relatively simple for users to verify whether their sensors come from the affected production batch. Here’s how to find your sensor serial number:
Immediate Verification of Sensor Status
Diabetic patient should check the serial number of any in-use or stored sensor by one of the following methods:
- FreeStyle Libre 3 app → Menu → About → Last 3 Sensors
- Libre 3 reader → Settings → System Status → System Info → Sensor SN & Status
- Physical inspection of the sensor carton or applicator label
Online Serial Number Lookup and Replacement
- Visit Abbott’s dedicated portal: https://www.FreeStyleCheck.com
- Enter the full serial number to determine eligibility for replacement.
Affected sensors qualify for immediate, no-cost replacement shipping.
Clinical Management While Awaiting Replacement
- Discontinue use of any confirmed or suspected affected sensor and dispose of it according to local biomedical waste regulations.
- Rely exclusively on blood glucose meter testing (fingerstick or reader-built-in meter) for all treatment decisions until an unaffected sensor is received.
- Continue usual sick-day rules and ketone testing if hyperglycemia is suspected.
Support Contact Information
- Telephone: 1-833-815-4273 (U.S., 8 a.m.–8 p.m. ET, 7 days/week)
- Live chat and additional resources: freestyle.abbott/us-en/support
Adverse Event Reporting
Healthcare professionals and patients are strongly encouraged to report any related injuries or malfunctions to:
- FDA MedWatch program or 1-800-FDA-1088)
- Abbott Diabetes Care directly
Diabetic Patient Sharing Stories on Forum Platform
Diabetes doesn’t wait for perfect technology, and many users of FreeStyle Libre 3 and Libre 3 Plus sensors are feeling the impact. Online communities like Reddit, Quora, and X reveal widespread concern over false low readings, unnecessary alarms, and disrupted sleep.
- On Reddit, one user wrote about being jolted awake at 3 a.m. by a 55 mg/dL alert, only to fingerstick at 120 mg/dL: “I’m losing confidence in this system… being woken up is annoying AF.”
- Another vented that sensors were “consistently reading hypo while I was nowhere near hypo,” causing unnecessary treatments and sleep deprivation.
- Quora users also questioned accuracy, with one noting frequent mismatches after logging 54 sensors: “Not at all.”
- Parents expressed worry about readings for young children, calling a 4-year-old’s results “proving very unreliable.”
Forum analysis shows false lows often spike in the first 24 hours and can persist, with discrepancies up to 100 mg/dL. Vitamin C intake (over 500 mg/day) may worsen errors. Despite frustration, the community shares tips on sensor placement, replacements, and FDA reporting, showing resilience amid unreliable technology.
Lessons Learned: The Importance of Glucose Monitor Safety
The Libre 3 recall is a reminder that medical devices, even widely trusted ones, can fail — with serious consequences.
- Regulation and Transparency: Early warnings from regulatory bodies and manufacturer responsiveness help prevent further harm.
- Patient Education: Users must understand CGM limitations and the need for redundancy (finger-prick testing).
- Vigilance and Reporting: Adverse events must be tracked and reported to improve safety.
- Shared Clinical Decision-Making: Healthcare providers should monitor CGM data and symptoms together, not rely solely on devices.
Summary Table of Key Facts
| Parameter | Detail |
|---|---|
| Total affected sensors (U.S.) | ~3 million |
| Serious adverse events (global) | 736 (57 in U.S.) |
| Reported deaths | 7 (all outside U.S.) |
| Defect | Falsely low glucose readings |
| Root cause status | Identified and corrected |
| Replacement availability | Immediate, free of charge |
| Official verification portal | www.FreeStyleCheck.com |
Conclusion
The link between malfunctioning glucose monitors and serious health complications, including seven possible deaths, serves as a sobering reminder that medical device reliability is paramount. For the approximately 1.5 million Americans currently using affected FreeStyle Libre 3 sensors, immediate action is necessary.
Check your sensor serial numbers today, request replacements if needed, and ensure you have backup glucose monitoring methods available. While continuous glucose monitors have revolutionised diabetes care, this incident demonstrates that vigilance and proper device management remain essential components of safe diabetes management.
Stay informed, stay safe, and remember that your health depends on accurate information—whether it comes from a sophisticated sensor or a simple finger-stick test.
Frequently Asked Questions
Can Freestyle Libre 3 false low readings be dangerous?
Yes. A false low reading can cause a user to treat a glucose level that is not actually low, potentially leading to true hypoglycemia from over-treating. This becomes particularly dangerous for users who rely solely on CGM data for insulin dosing decisions.
Can the Freestyle Libre 3 cause severe health events due to false low readings?
False lows can lead to incorrect insulin dosing, unnecessary carbohydrate intake, or panic responses. While the device itself does not directly cause death, mislabeled low glucose readings that lead to incorrect treatment decisions may contribute to severe events such as actual hypoglycemia.
What causes false low readings on the Freestyle Libre 3?
Common causes include: compression lows (lying on the sensor during sleep), poor sensor placement (lean areas, scar tissue, muscle pressure), sensor acclimation period (first 12–24 hours), dehydration or poor circulation, rapid glucose changes, expired or damaged sensors, and medication interference, especially high-dose vitamin C.
What is a compression low, and can it affect Libre 3 accuracy?
A compression low happens when pressure is applied to the sensor—usually while sleeping on one side. It reduces blood flow in the surrounding tissue, causing the sensor to report an artificially low reading.
Can dehydration cause the Libre 3 to show false low readings?
Yes. Dehydration reduces interstitial fluid volume, making CGM readings less reliable. This may cause artificially low or fluctuating values. Staying hydrated helps improve sensor performance.
Does vitamin C affect Libre 3 readings?
High doses of Vitamin C (500–1000 mg) can interfere with Libre 3 readings, potentially raising CGM values by 50–100 mg/dL or causing inaccurately low or high readings depending on dose and timing. Users should review recommended limits in the device manual.
What should I do if my Libre 3 repeatedly shows false low readings?
Follow these steps:
1. Check for compression or pressure on the sensor.
2. Wash your hands and verify with a fingerstick meter.
3. Ensure the sensor is properly inserted on FDA-approved sites.
4. Hydrate and wait 10–15 minutes if dehydration is suspected.
5. Replace the sensor if errors persist or readings remain inconsistent.







Write a comment
Your email address will not be published. All fields are required